By Paul Fraumeni
It was 1983.
Janet Rossant was 33 years old.
Six years earlier, she left her native England with degrees from Oxford and Cambridge. She had landed a position at Brock University, across Lake Ontario from Toronto, in St. Catharines.
An assistant professor of biology, she headed a laboratory team seeking to understand how the embryo develops. To do that, the Rossant team was starting to develop transgenic mice.
“This was a new field of developmental biology, and we were excited by the possibility of being able to add genes to embryos and study their effect on development. But to do this research, we needed specialized equipment and we also needed the money to buy it,” she says.
Rossant applied to the Banting Research Foundation (BRF), which awarded her $25,000 — not a huge amount, but an important influx of financing to a researcher starting to build a track record.
“People like to talk about the tipping point,” she says. “When you’re a young investigator and you don’t have a lot of funding, it’s hard to move to the next level. So, the Banting Foundation was then and still is a really important support for new investigators, providing that critical piece of funding just when you need it.”
That early support paid off for Rossant — and for global society.
By 1985, Rossant had moved to the University of Toronto and the Samuel Lunenfeld Research Institute (known today as the Lunenfeld-Tanenbaum Research Institute) at Mount Sinai Hospital, only two years after receiving the BRF grant.
Over the next 30 years, Rossant — who is now the President and Scientific Director of the Gairdner Foundation — conducted research that earned her renown and a bevy of awards for her work on genetics and stem cells.
Today, Rossant is a University Professor in the Department of Molecular Genetics at the Temerty Faculty of Medicine, and a Senior Scientist and Chief of Research Emeritus at the Hospital for Sick Children.
So, how does the discovery of insulin decades before relate to Rossant’s work?
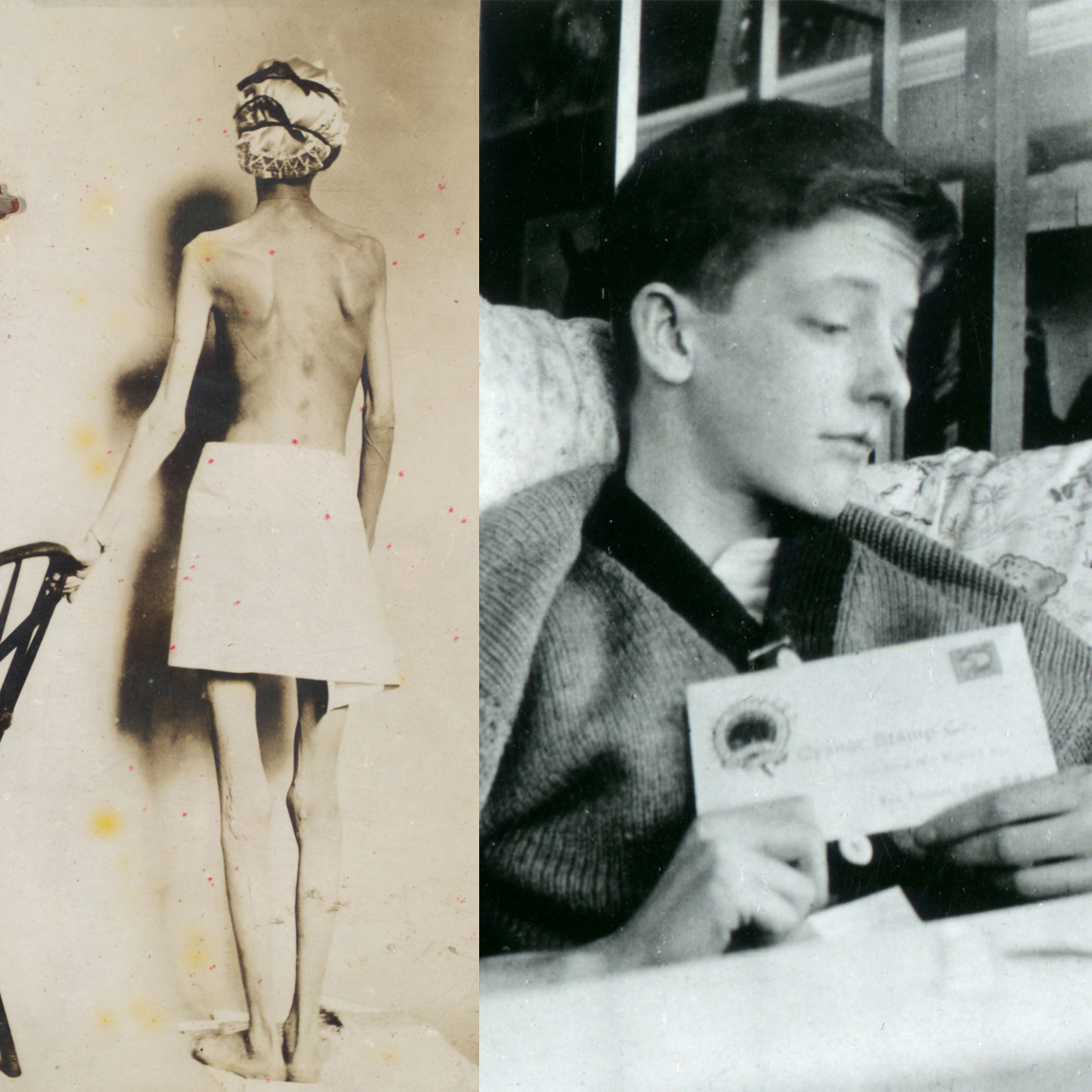
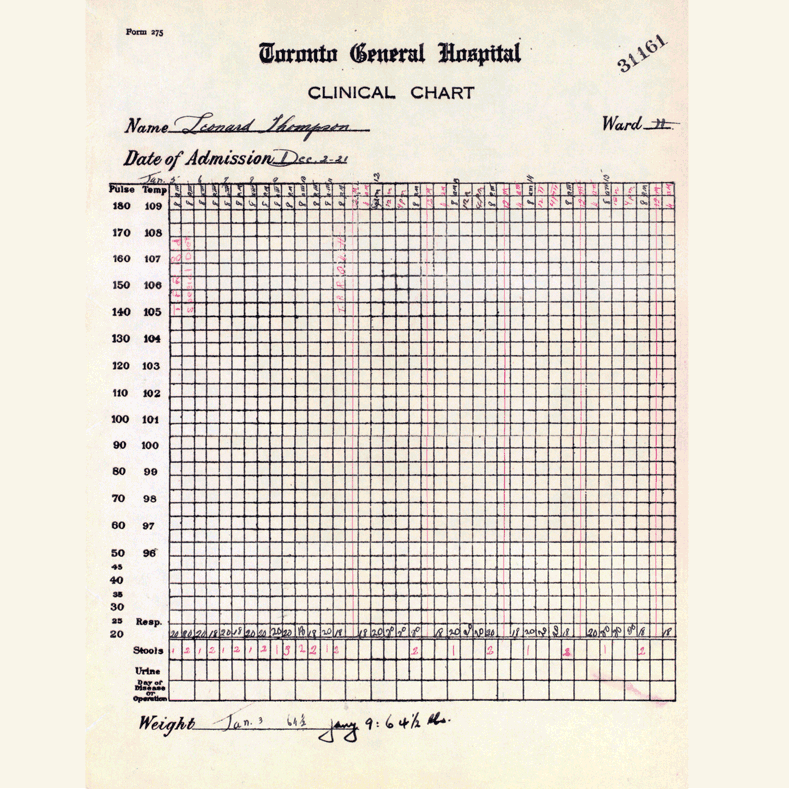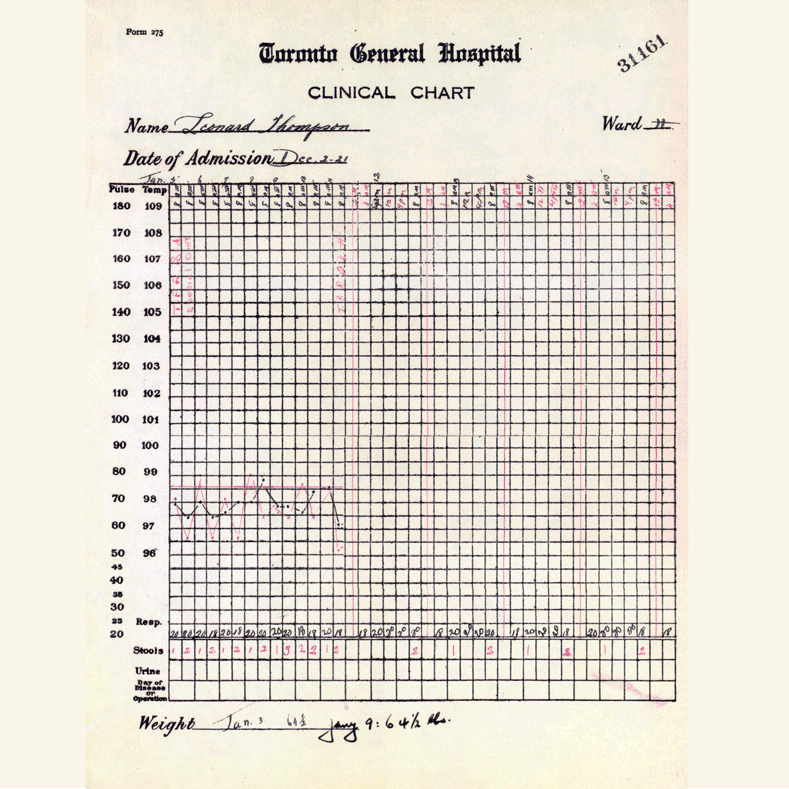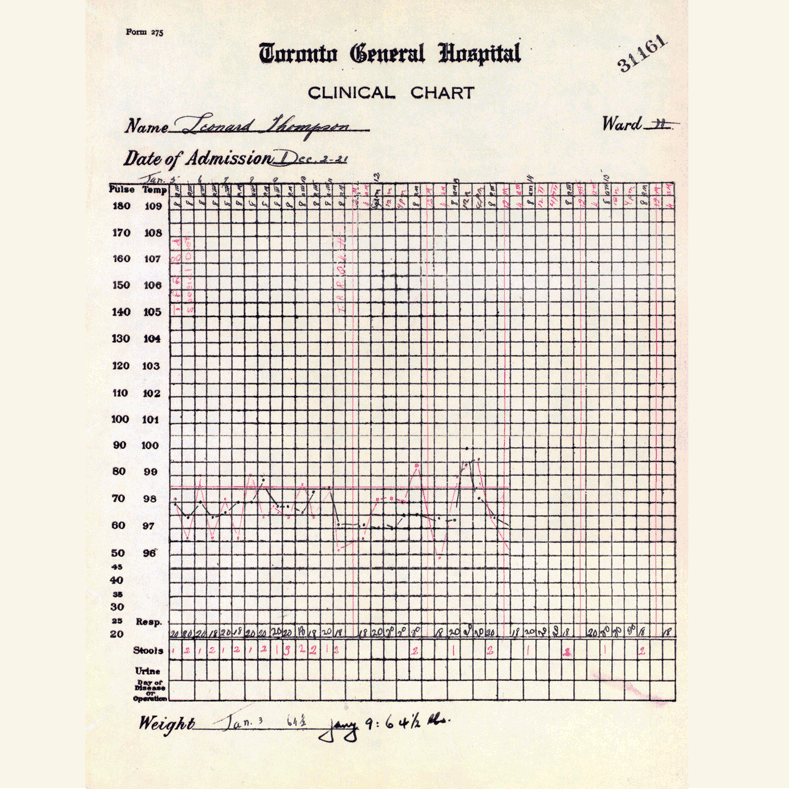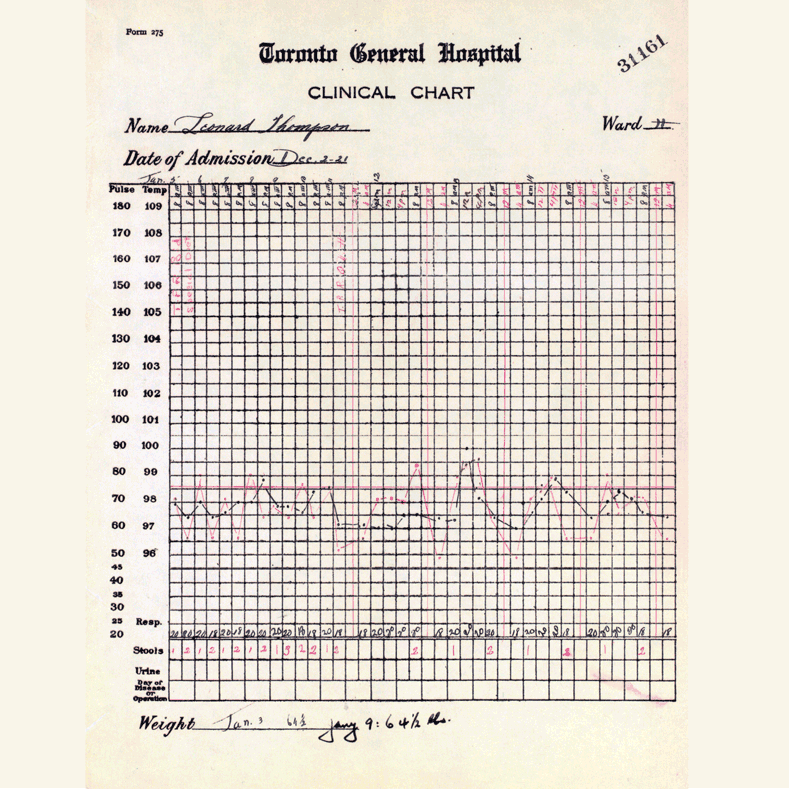
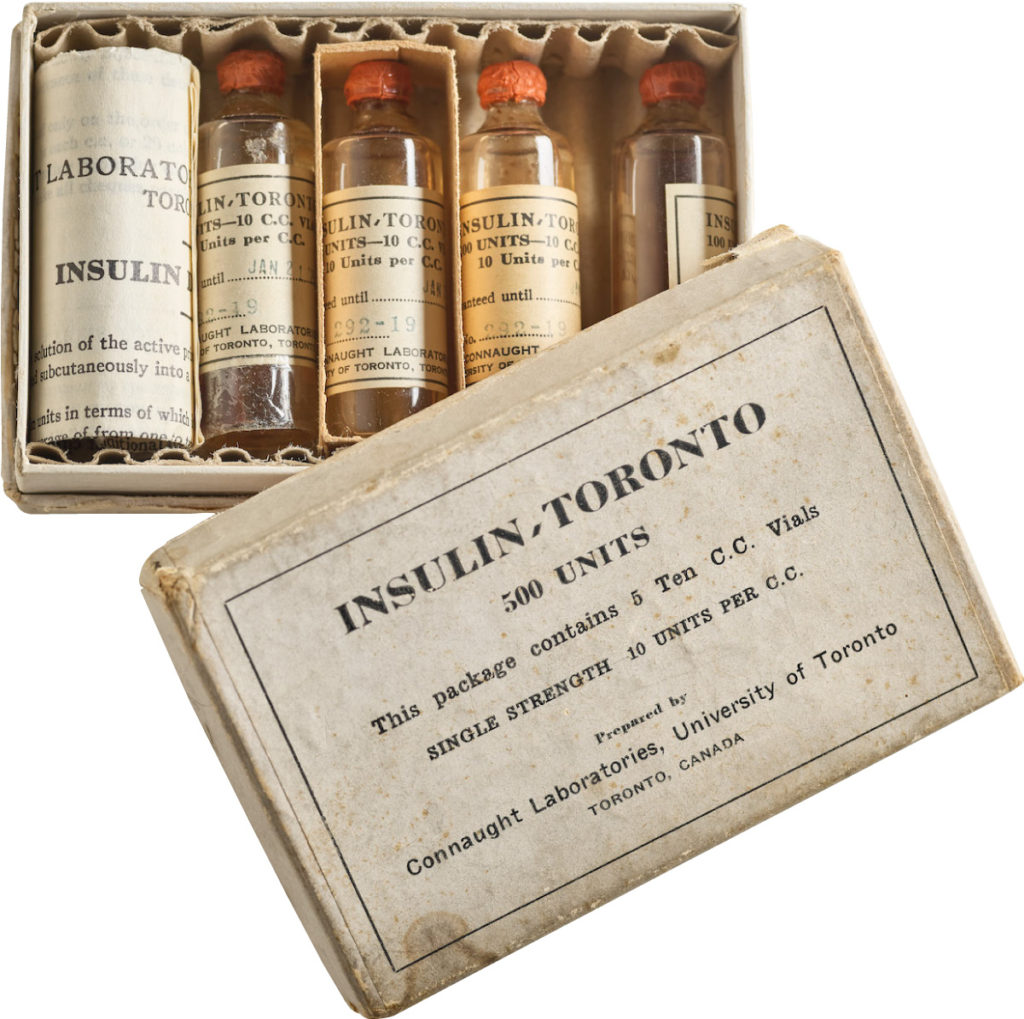
In 1921, sixty-three years before Rossant applied for that grant, an unlikely team was hard at work in a U of T lab on a disease — diabetes — that had killed millions over the centuries and continued to bewilder scientists.
Frederick Banting (MD 1916), a GP with little research experience, and Charles Best, a medical student, were working from an idea Banting had about how to isolate the mysterious secretion of the pancreas that controlled metabolism.
U of T’s then chair of physiology, Professor J.J.R. Macleod, somewhat reluctantly gave the team lab space, equipment and dogs to use for testing.
Banting’s idea worked.
The discovery won the Nobel Prize and continues to enable people with type 1 diabetes to live full, rich lives — an unthinkable outcome prior to the team getting down to work in 1921.
That innovation would have been quite enough.
However, it has acted as a catalyst to stimulate a century of medical research in a dizzying array of areas that continue to have an enduring impact. And, an important generator of that research came from the creation of two foundations established in the spirit of Banting and Best.
A fundraising campaign that raised $500,000 founded the BRF in 1925. A large bequest in 1948 added to the endowment.
“Our mission is to fund young investigators, the future Bantings and Bests, in the way they were funded by Macleod, at the beginnings of their careers when they have a bold idea that just might move society forward,” says Professor Catharine Whiteside (BSc ’72, PhD ’84, MD ’75, PGME ’83), BRF chair, Former Dean of Medicine and an Emerita Professor with the Department of Medicine.
Then in 1960, W. Garfield Weston Foundation funding established the Dr. Charles H. Best Foundation. The original idea was to support research at the discretion of Professor Best (MD ’25), who went on to conduct important studies in a number of areas and become a U of T research leader.
When Best retired in 1965, the funds were designated to U of T’s Banting and Best Department of Medical Research. That unit became part of the Donnelly Centre for Cellular and Biomolecular Research at U of T.
“Today, those funds are used to support researchers getting started on their careers through a program called the Dr. Charles H. Best Postdoctoral Fellows,” explains Professor Peter Lewis, the program’s Board Chair and former Chair of the Department of Biochemistry at U of T. “This funding enables us to help researchers of a high calibre from around the world to pursue their ideas and to learn with the scientists of the Donnelly.”
These two foundations have helped to start remarkable careers.
“The influence of Banting and Best goes way beyond diabetes and insulin,” says Professor Reinhart Reithmeier, also a former Chair of the Department of Biochemistry at U of T and winner of a Best Postdoctoral Fellowship when he was a U of T fellow in the late 1970s.
Indeed, early BRF recipients comprise a community of young researchers who went on to be superstars. Among them was Gordon Murray (MD 1921), who the BRF awarded funding from 1937 to 1939.
He was one of the first researchers in the world to show how the drug heparin is effective in preventing thrombosis and embolism. Murray also developed the first artificial kidney to be used successfully in North America.
A short time later came William Mustard (MD ’37), who the BRF awarded funding to in 1950. He created the “Mustard Operation” that is still used worldwide. The surgery corrects a complex defect in “blue babies” — infants born with the arteries and veins to and from the heart connecting with the wrong heart chambers.
Then there was Henry Friesen (Hon ScD ’00), awarded funding from 1972 to 1973, whose research on human growth hormone made possible successful replacement therapy in hormone-deficient children.
The Toronto insulin discovery continues to ignite important ideas from newer generations.
At the Donnelly, the 2019 Best Postdoctoral Fellow Juline Poirson is studying the ubiquitin-proteasome system (UPS). It is critical in ensuring the normal functioning of cells, notably by destroying proteins that are no longer needed. But in diseases such as cancer, the UPS is dysregulated.
Poirson is working to understand why this happens with certain proteins. Her work could lead to important drug developments to treat diseases like cancer.
“I came from France to the Donnelly because I knew it would help me build on what I had already learned,” she says. “It is one of the best research centres in the world when you work on protein-protein interaction. This experience is going to help me for the rest of my career.”
At York University, Professor Ali Abdul-Sater (U of T Postdoctoral Fellow ’16) is an assistant professor in the Faculty of Health.
BRF funded Abdul-Sater in 2018, enabling him to delve deeper into his focus: how abnormal inflammation is at the core of rheumatoid arthritis.
His specific interest is in exploring how TRAF1, an immune signalling molecule, can control inflammation. Abdul-Sater’s team has proven that people with a genetic variation in their TRAF1 make less TRAF1 protein, which increases their risk of developing rheumatoid arthritis.
But adjusting or removing TRAF1 is dangerous because the protein plays other vital roles in our immune system. Finding the solution is Abdul-Sater’s next research project.
“I wouldn’t have come this far without the Banting support,” he says. “As a new investigator, I couldn’t get funding because I couldn’t get the funding I needed to do certain experiments in the area of inflammation. It was a catch-22 predicament. Our work now has close to $2 million in grants. It all started with the Banting funding.”
At SickKids Hospital, Professor Nomazulu Dlamini (PGME ’11) is an associate professor of pediatrics at the Temerty Faculty of Medicine. The pediatric neurologist and scientist specializes in understanding and treating strokes in children.
Her focus is dystonia, a disabling and painful disorder that can occur in children who have experienced a stroke. It’s characterized by involuntary, repetitive muscle contractions, twisting movements and abnormal posturing. Often, it is resistant to treatment.
It is thought that the problem originates in the basal ganglia, a network in the brain. Dlamini’s lab is studying the differences in the neural network between childhood stroke patients who have dystonia and those who don’t. Understanding why some children experience dystonia will increase the potential of developing effective therapies.
The BRF awarded Dlamini $25,000 in 2018 to support her work in understanding dystonia.
“That support has been very helpful. With the pilot data from the work we have been able to get because of the Banting funding, we’ve been able to leverage that for further funding,” says Dlamini. “That’s all because Banting believed in our idea of discovering why there is this difference between these two groups of children.”
Which researchers will the two foundations support next?
That remains to be seen, but Rossant, who was Chief of Research at SickKids for 10 years, is adamant that seed funding for researchers at the beginning of their careers is vital in creating new knowledge and applications that will improve health.
“My most important duty at SickKids was to encourage researchers, particularly young researchers, to help them set up their careers with equipment, infrastructure and mentoring, and provide them with a collaborative environment in which they could grow,” she says.
“And that meant helping them get the money they needed, which is why these foundations are so important. They are focused solely on enabling young researchers to get started.”
Reithmeier emphasizes that the model the Banting and Best foundations use today is the same type of support U of T provided for insulin research in 1921 — getting young researchers the funding they need to pursue their curiosity.
“Frederick Banting had a great idea. Actually, it was a crazy idea,” says Reithmeier. “But, those are the ones that we have to fund.” •